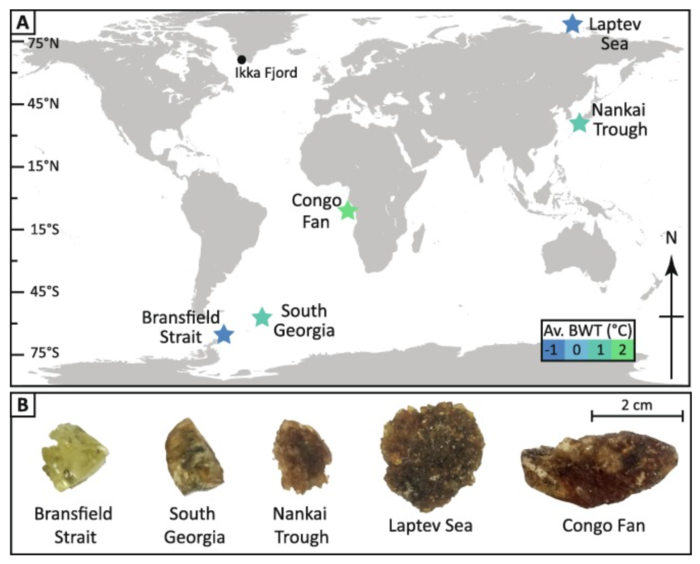
Few studies examine natural ikaite (Ca(CO3)•6(H2O)) because it is so difficult to work with without it decomposing before you undertake the experiments on it. We were able to access a number of marine ikaites sampled from all over the world, including from Antarctica, Siberia, and Africa. The ikaites were frozen immediately upon collection, to stop them decomposing. Then, to transport the ikaites between collection sites and laboratories, they were carried in a thermos flask that had been pre-frozen and packed with ice. Despite warm weather and long delays during travelling, our samples made it intact to the lab (Fig. 1a&b).
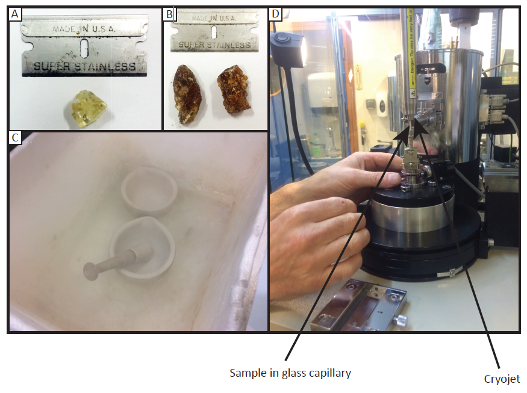
The most interesting question we wanted to answer, what ikaite breaks down to, we tried to find out using stepped heating in a powder X-ray diffractometer (PXRD), which can tell us the crystal structure of a sample. Yet, in order to use this instrument, the ikaites had to first be powdered without it getting too hot and transforming. So, we set up a “cold box” – a polystyrene box with liquid nitrogen (-40 °C) in, in which we put the mortar and pestle to grind the ikaites (Fig. 1c). Then the powder was put into capillaries (still in the cold box and using giant gloves to prevent cold burns), and mounted under a cryojet (a pipe blowing freezing cold air onto the sample, Fig. 1d). We also put one small sample into the scanning electron microscope to watch it transform at 21 C (Fig. 2).
Our experiments, as published in Vickers et al. (2022) show that, unlike previous studies on synthetic (i.e. laboratory-made) ikaite, natural ikaite transforms directly to calcite, without any intermediate phases, via a quasi- solid-state transformation. Our study therefore shows that laboratory-made ikaite may not be representative of the diverse ikaites found in nature, and our experiments mostly show that further studies are needed to really understand the behaviour of this strange mineral.

Publication details: Vickers, M.L., Vickers, M., Rickaby, R.E., Wu, H., Bernasconi, S.M., Ullmann, C.V., Bohrmann, G., Spielhagen, R.F., Kassens, H., Schultz, B.P. and Alwmark, C., 2022. The ikaite to calcite transformation: implications for palaeoclimate studies. Geochimica et Cosmochimica Acta, 334, 201–216. https://doi.org/10.1016/j.gca.2022.08.001