Some examples of the significance of chirality for the biological activity of drugs will be given. Biocatalysis is the use of biological catalysts, such as pure enzymes, bacteria or fungi, as catalysts in chemical reactions. Their advantages in both resolution and asymmetric synthesis will be demonstrated.
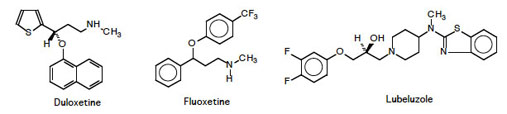
Results from the Biocatalysis Group at NTNU using in particular lipase B from Candida antarctica, CALB, demonstrates the possibilities and limitations of this enzyme. This is related to the 3D structure which explains the enantioselectivity, E, in resolution reactions. Surprisingly, it has been shown that the E-value changes during resolutions.CALB has been used to synthesise enantiopure drugs such as Duloxetine, Fluoxetine (non-tricylic antidepressants), Lubeluzole and a precursor for Atorvastatin.
More recently, acetyl xylan esterase from Bacillus pumilis has been shown to be selective for hydrolysis of carbohydrate acetates. The 4 stereoisomers of a branched alditol have been synthesised. They are shown to be significant for understanding the origin for aerosols present over The Amazonian rain forest.
Our thorough investigation of the catalytic effect of porcine pancreas lipase in desymmetrisations has revealed the presence of enzymes with opposite stereoselectivity.