Information about this microscope
This confocal microscope is set up for live cell imaging and allows efficient detection of fluorescence with a high sensitivity, which minimizes the damage to living cells. A confocal has improved image quality because it filters information from out-of-plane light sources. Samples can be imaged in layers by moving the focal plane in precise steps through it with the IX81 motorized z-drive objective movers. It is possible to take several images per second or for long-term experiments with longer time-intervals.
The FV1000 has a unique SIM Scanner that incorporates two independent, fully synchronized laser scanners in one single compact design. This makes it possible to, with one laser, bleach or activate, and do simultaneously high resolution confocal imaging with the other beam. The SIM scanner makes the FV1000 the most suitable confocal microscope for a variety of applications (see below), including FRAP, FLIP, photoactivation, FRET, uncaging, laser ablation, etc.
Spesifications
- Heating chamber
- CO2 controller
- SIM scanning: one laser beam stimulates, while a second laser simultaneously provides high-resolution imaging. Synchronization of these two functions ensures that the rapid cellular reactions that occur during or immediately following stimulation can be detected and imaged. An arbitrary region of interest can be specified for stimulation and scanning independently, with unrestricted control of variations in timing, duration and intensity.
- Tornado scanning: increases photobleaching efficiency. Conventional reciprocating scanning systems tend to be slow, and there are cases where photobleaching is inadequate. In contrast, with tornado scanning (which requires a SIM), photobleaching is much more efficient.
Lasers
- 405nm
- Multi-line Argon laser: 457nm, 488nm and 515nm
- 559nm
- 635nm
Objectives
|
Magnification |
Type |
Numerical Aperture |
Working distance(mm) |
Immersion |
|---|---|---|---|---|
|
20x |
UplanApo |
0.7 |
0.65 |
- |
|
40x |
Semi-Apo |
0.85 |
0.2 |
- |
|
60x |
PlanApo |
1.1 |
0.13 |
Oil |
|
100x |
UPlanFLN |
1.3 |
0.2 |
Oil |
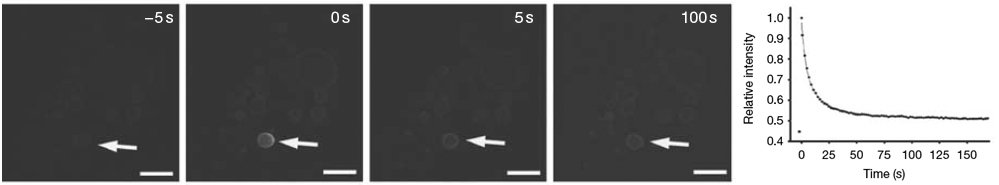
Fluorescence Recovery After Photobleaching (FRAP)
This technique is very useful in biological studies of cell membrane diffusion and protein binding. Similar techniques have been developed to investigate the 3D diffusion and binding of molecules inside the cell.
In FRAP, a very small, selected region is subjected to intense illumination, usually with a laser, to produce complete photobleaching of fluorophores in the region. The result is a dramatic reduction or annihilation of fluorescence and the molecules are irreversibly photobleached. After the photobleaching pulse, the rate and extent of fluorescence intensity recovery in the bleached region is monitored as a function of time. The movement of unbleached molecules from surrounding areas and into the bleached is measured by time-lapse microscopy.
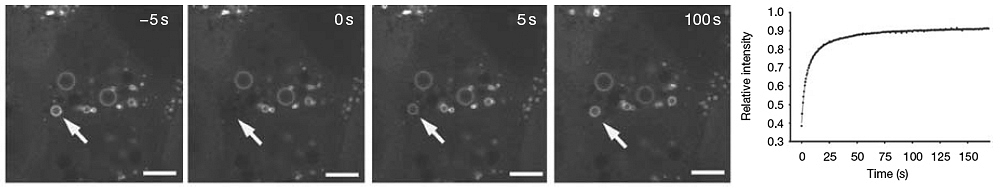
Photoactivation
Genetically encoded fluorescent proteins that generally display little or no initial fluorescence under excitation at the imaging wavelength, but dramatically increase their fluorescence intensity after activation by laser wavelength, are termed photoactivatable. Among the proteins that demonstrate photoactivation, a green fluorescent protein variant, termed PA-GFP, has been the most widely studied. The movement of activated molecules from one activated area to the surroundings can be measured and plotted over time.
Fluorescence Resonance Energy Transfer (FRET)
An adaptation of the resonance energy transfer phenomenon for fluorescence microscopy in order to obtain quantitative temporal and spatial information about the binding and interaction of proteins, lipids, enzymes, and nucleic acids in living cells. FRET microscopy is performed using either steady state or time-resolved techniques, but time-resolved FRET imaging has the advantage of more accurately mapping the donor-acceptor distance. A standard fluorescence microscope equipped with the proper excitation and emission filters and a sensitive video camera can be used to perform FRET imaging.
Fluorescence Loss in Photobleaching (FLIP)
A method where loss of fluorescent signal is measured. A defined region within a living cell is subjected and continuously bleached. Over a measured time period, if the fluorescent molecules can diffuse into the area that is bleached, loss of fluorescence throughout the cell will occur. By calculating the rate at which fluorescence is extinguished from the entire cell, the diffusional mobility of the target fluorophore can be determined.