From cartilage and into the brain – how a cartilage protein blocks brain plasticity
How our mind can develop and acquire new knowledge, while at the same time preserve memories across a lifetime has been a longstanding question within neuroscience. The brain is extraordinary in its adaption to our experiences. This feature, termed plasticity, is crucial in learning and memory formation. However, insufficient levels of plasticity are a major problem in recovery from brain injury. A key element in regulating brain plasticity has turned out to be a matrix protein structure outside the neurons termed the perineuronal net, which is made up of a meshwork of proteins and sugars that enwraps the surface of certain neurons.
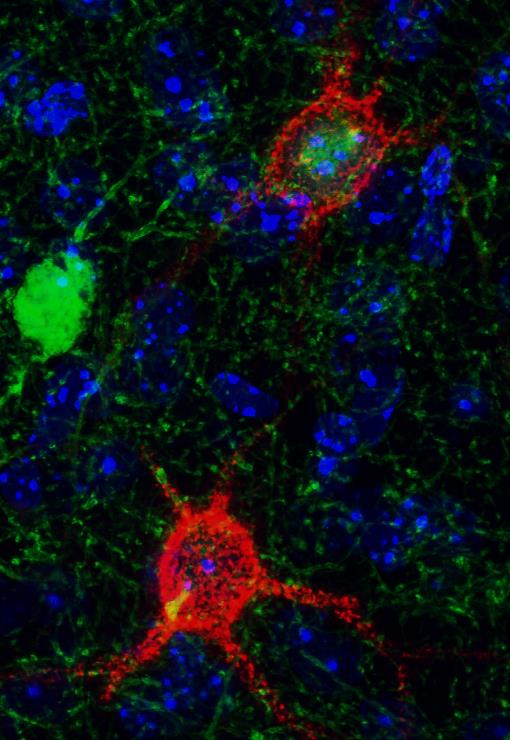
A joint collaboration between researchers from the University of Oslo, Norway, and the University of Cambridge, UK, have shown that a single component of the perineuronal net, the main component of cartilage called aggrecan, is an essential regulator of brain plasticity.
Without aggrecan, the perineuronal nets never form and the brain stays in a high plasticity state throughout life. If aggrecan is removed late in life, the nets disappear and the brain returns to a state of high plasticity similar to that of a young brain in development.
The new research, published in The Journal of Neuroscience, shows that aggrecan is an essential regulator of brain plasticity through its role as the backbone of the perineuronal net. These findings and the methods developed in the study will allow us to address several critical research questions about brain function, potentially leading to new treatments for neurological diseases.
Background:
Why study a matrix protein in the brain?
First discovered in the late 1800s, the perineuronal net is a structure with distinct features that wraps densely around several neurons in the brain. While supporting evidence show that the perineuronal net restrict plasticity, it has proven difficult to determine the key molecular element in the formation of the structure. Aggrecan is the largest component of the perineuronal net and believed to be the backbone for the whole structure. Outside the brain, aggrecan is the main protein in cartilage. The protein carries long chains of hundreds to thousands of sugar molecules, forming an ideal structure for the shock absorbing and structural properties of cartilage matrix. Due to its role in cartilage, removing aggrecan from all tissues is lethal. The researchers therefore developed a novel transgenic mouse line where the gene coding for the aggrecan protein can be removed in specific tissues, such as neurons in the brain. The novel mouse line will be a valuable tool in studying matrix-dependent plasticity throughout the brain.
Resetting the brain to a juvenile “high-plasticity” state
After establishing that genetic removal of aggrecan prevented formation of perineuronal nets, Dr. G. Dick in the team led by J. Fawcett brought the mouse to University of Oslo and the team led by Marianne Fyhn and Torkel Hafting, where K. Lensjø and T.Dinh first investigated how this affected plasticity in the visual system of adult mice. The results were quite striking.
“It has long been known that the visual system undergoes a period of heightened plasticity in juveniles” explains Lensjø, “wherein if vision of one eye is restricted for a short period, the brain will change and nearly stop responding to signals from the closed eye, while the response from the open eye becomes stronger. This is not the case in adults because plasticity is limited. After aggrecan removal in adults, however, just a few days of vision deprivation caused similar effects as in juvenile mice, suggesting that we were resetting brain plasticity to a juvenile state - even the mechanism for how the plasticity happens seems to be the same as in juvenile mice”.
Improved memory and possibility for developing new disease treatments
D. Rowlands and S. Yang from the Cambridge team then went on to investigate how aggrecan removal would affect how well the mice could distinguish, or remember, novel from familiar objects. Mice lacking aggrecan did better than control animals, presumably because increased plasticity allowed them to learn to recognize the objects faster.
Taken together, the results suggest that removing aggrecan dramatically increases plasticity in adults. While tempting to think that this may be a highly beneficial feature for humans, high brain plasticity may also destabilize the brain. “The reduction in plasticity when the nets mature is important to preserve the neural pathways for learnt events or skills. Memories can maintain over years and decades, and it is likely that the perineuronal nets are important for such storage too” says Dick. The Oslo team has recently also shown that removing the nets destabilizes remote memories. Furthermore, loss of the nets is associated with some neurological diseases in humans, such as schizophrenia. “It is only when we understand the underlying molecular components and their interactions we can truly begin to understand these diseases and come up with treatments tailored to the individual”, says Fyhn “These results are a stepping-stone to start untangling how the perineuronal nets work at the molecular level”.