About the project
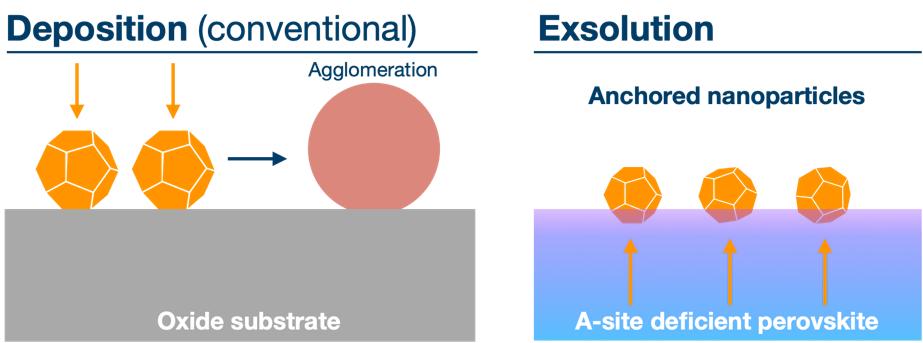
Solid-state electrochemical devices play a crucial role in energy conversion and storage of renewable energy. The performance of such electrochemical cells is limited by the electrocatalytic activity of the electrode. Here, catalytic metal nanoparticles can be applied, but they tend to agglomerate over time which reduces their performance (left figure).
Exsolution is a growth phenomenon where metal nanoparticles segregate from an oxide material but remain embedded at the surface (right figure). The anchored nature of the nanoparticles hinders agglomeration and is one of the reasons for why such nanoparticles are observed to have increased performance as electrodes.
Objectives
Most exsolution studies so far convey empirical knowledge of individual materials systems, and there are many unresolved questions on the exact underlying mechanisms involved in exsolution. The main objective of the project is to find generic principles of the exsolution process which are transferable across different materials systems. In collaboration with the Solaris initiative at UiO: Energy and Environment, a combinatorial synthesis route using pulsed laser deposition (PLD) is applied together with high-throughput characterization methods to explore a large parameter space of material compositions. Choice of parameter space builds upon insight from atomistic simulations of the energy landscape of the majority defects in the material, in addition to the composition’s technological relevance. The project collaborates with the Electrochemistry group for efficiently applying the new insight to device development for an electrochemical cell.
Financing and cooperation
The project builds on cross disciplinary collaboration between chemists and physicists in various groups at the center for materials science and nanotechnology. The project is funded by The Faculty of Mathematics and Natural Sciences, University of Oslo.
Methods:
-
Synthesis: Combinatorial pulsed laser deposition
-
Characterization: Scanning electron microscopy, x-ray diffraction, transmission electron microscopy, atomic force microscopy, thermogravimetry
-
Analyses: Automated analyses of hyperspectral and large (> GB) datasets
-
Modelling: Density functional theory